Why Is Activated Liquid Zeolite Better?
Activated Liquid Zeolite is the term used when zeolite (clinoptilolite) has undergone a cleansing process to remove any and all impurities from the zeolite cage, and then a micronisation process so the zeolite particles can be absorbed into the blood stream.
Zeolite is its raw form is widely used around the world for water purification.
How does it work?
Clinoptilolite (zeolite) has a cage-like structure, with pores and channels running through the crystal.
The cage carries a net negative charge, making it one of the few negatively-charged minerals found in nature.
Because of its cage-like structure and negative charge, clinoptilolite has the ability to draw to itself and trap within itself positively charged heavy metals and other toxic substances.
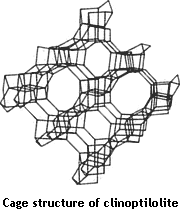
The zeolite like in the Daily Detox Liquid Zeolite attracts and traps small, highly-charged particles that fit into the pores and channels of the zeolite cage.
The SiO4 units are electrically neutral, but each AlO4 unit carries a negative charge, creating fixed, negatively charged sites throughout the crystal structure.
The negative charges of the AlO4 units are balanced by the presence of exchangeable, positively charged metals known as cations (pronounced CAT- ons).
These cations usually consist of calcium, magnesium, sodium, potassium and iron.
These ions are only loosely held and can be readily displaced by other substances, such as toxic heavy metals.
This phenomenon is known as cationic exchange, and it is the very high cationic exchange capacity of zeolites which provides for many of their useful properties.
In their chemical makeup, zeolites are a lot like clay, in that they are both made up of aluminum, silica and oxygen.
However, there is an important difference in their structure.
Many types of clay have a layered crystalline structure (similar to a deck of cards) and are subject to shrinking and swelling as water is absorbed and removed between the layers. In contrast, zeolites have a rigid, 3-dimensional crystalline structure (similar to a honeycomb) consisting of a network of interconnected tunnels and cages.
Water moves freely in and out of these pores but the zeolites framework remains rigid.
Another special aspect of this structure is that the pore and channel sizes are nearly uniform, allowing the crystal to act as a molecular sieve.
Does clinoptilolite remove good minerals?
Whereas most chelating agents used for detoxification are non-specific, only relying on charge for binding potential, the clinoptilolite seems to be highly specific for the toxic heavy metals.
Research has shown that the smaller the diameter of the metal and the higher the charge of the metal, the greater the affinity it has for the activated liquid zeolite.
Higher charges simply increase the strength of binding with higher binding characteristics.
The small size allows for deeper access into the zeolite pores with more points of coordination (attachment).
Larger atoms do not fit into the zeolite cage as well and so are more easily exchanged for higher-affinity metals.
As an example of this phenomenon, arsenic has a charge of +3 and an atomic radius of approximately 1.8 angstroms, while potassium has a charge of only +1 and an atomic radius of approximately 2.8 angstroms.
The arsenic binds with very high affinity for the zeolite while the potassium has no affinity whatsoever.
It just so happens that the most toxic metals are those with a small radius and high ionic charges.
The healthy minerals and electrolytes tend to have larger size with smaller ionic charges.
The clinoptilolite binds a variety of toxins including heavy metals (Lead, Cadmium, Mercury, etc..), nitrosamines and others.
Cationic exchange is an entirely passive process when the zeolite is in close proximity to these high-affinity compounds, they will be drawn to the zeolite and either absorbed into the cage or adsorbed onto the surface of the zeolite.
There is no chemical activity in this process. The zeolite will not be drawn to compounds in an effort to ‘rip’ metals away from them.
In other words, the zeolite will not pull metals that are sequestered inside tissue or bone.
If, on the other hand, the tissue has already released free metals into the system, the zeolite will have the ability to trap and remove it.
Related articles
Activated Liquid Zeolite Benefits
The Activation Process of Liquid Zeolite
Comparing Powdered & Liquid Zeolite
Occupational Exposure to Heavy Metals
Your Exposure to Cadmium & Arsenic
Your Exposure to Lead & Aluminum
Your Exposure to Copper & Zinc
Dr. Stewart Lonky – Medical Complications From Toxins AUDIO PRESENTATION



