The Science Behind Crystal Energy & MegaHydrate
When you consider how hard or soft ordinary water (tap water, bottled water, tank water etc.) is, you’ll more than likely find that most of the water available has a high surface tension, as well as what is called a high wetting angle.
Basically this means it’ll have a difficult time wetting the cells within the body.
You see within the cells of your body (your cell membranes), are phospholipids (fats), which have a low surface tension of around 45 dynes/cm.
Cells require a surface tension of about the same range for water to wet the cells, thus passing through the fatty acid cell wall carrying nutrients etc..
This concept is the same relationship as the idea that oil and water do not mix.
Normal tap water with a surface tension of around 70 – 75 dynes/cm is able to suspend the cells in the body, but is not in intimate contact with the surface of the cell, and passes through the body not being fully utilised by the cells.
High surface tension water molecules can actually promote dehydration due to the required donation of ions from already within the body to facilitate the necessary process from hard to soft.
The lower the surface tension, the lower the wetting angle and the “wetter” the water is.
Surface tension explained
Surface tension is the force that exists between a liquid and the atmosphere it is in.
For example, in atmospheric air, a drop of water will bead up on some solid surfaces.
It is the surface tension existing between the water and the air that allows this to occur.
The drop of water can spread, or wet-out, on another solid surface if the new surface has molecular forces (surface energy) high enough to overcome the water/air surface tension and draw the water flat on to it.
Can the surface tension of water be improved?
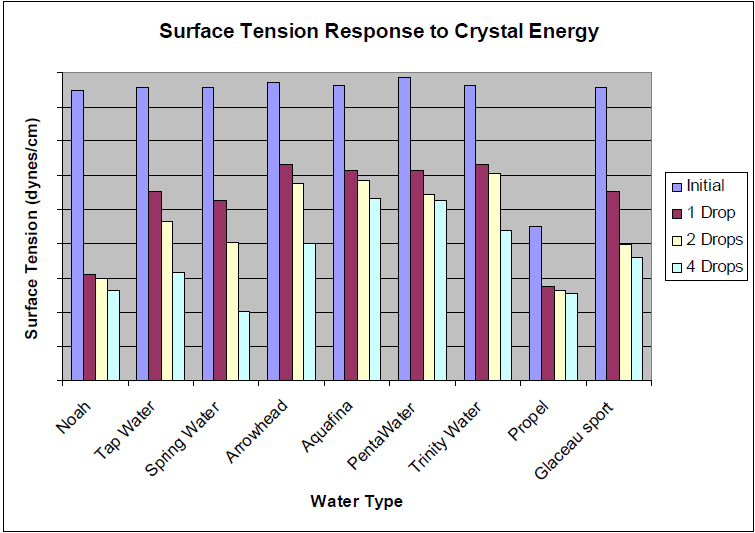
Extensive research has indicated that water exhibiting low surface tension characteristics is predominantly sourced from locations where the water traverses geological formations rich in high concentrations of silicate minerals.
These findings suggest that the interaction between water and silicate-rich earth results in a modification of the water’s physical properties, particularly affecting its surface tension.
A notable example of this phenomenon can be observed in products like Crystal Energy, which contains silica colloids that exist in a microcluster formation.
These microclusters possess unique properties that enable them to interact with water at a molecular level.
The introduction of such silica colloids into drinking water can lead to significant alterations in its structural composition, which in turn contributes to a measurable decrease in surface tension.
This reduction in surface tension can enhance the water’s ability to penetrate and hydrate tissues, providing potential benefits for overall hydration and absorption in biological systems.
Wetting tension
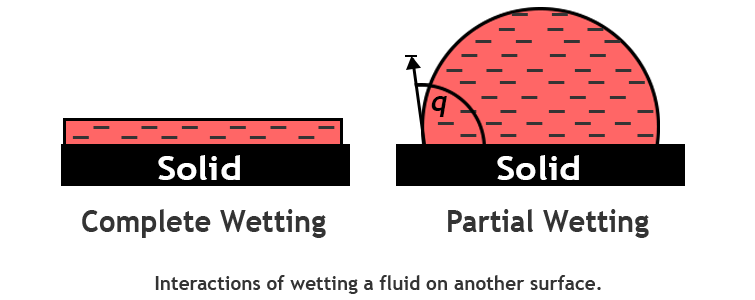
Wetting tension is the maximum liquid surface tension that will spread, rather than bead up, on the film surface.
It is a measurable property that estimates a film’s surface energy.
Wetting tension is determined by applying different test solutions of increasing surface tensions until one is found that just spreads (wets) the film surface.
Units are dynes/cm.
Contact angle
Contact angle is a measurement of the behavior of pure water in contact with the film surface.
The surface energy of the film controls whether the droplet tends to stand up or flatten out.
This is quantified by measuring the contact angle of the droplet with the surface.
A higher energy film will cause the droplet to be flatter and closer to the surface, which results in a smaller contact angle value.
With a lower surface tension and a low wetting angle, cells become fully hydrated.
Hydration is crucial for energy production
Dehydrated cells metabolise in a catabolic state, which means the body starts utilising its own tissue for energy production, resulting in degeneration of cellular health and immune response.
Remember this! The wetter your body, the greater the fluidity you’ll be able to experience… in your body.
Related products


Experience the difference adding MegaHydrate or Crystal Energy to the water you drink today.
Related articles
MegaHydrate ORP Test Over 6 Hours
What’s the Difference Between Crystal Energy and MegaHydrate
Crystal Energy Improves Absorption
Understanding ORP & How It Affects You
Crystal Energy Softens Water Tension
MegaHydrate / Crystal Energy – Wetter Water
I Drink Water and Still Feel Thirsty
DISCLAIMER: The products and information mentioned on this site are not intended to diagnose, treat, cure, or prevent any disease. Information and statements made are for education purposes and are not intended to replace the advice of your medical professional.



