What is pH?
The term pH stands for the hydrogen ion concentration of a solution – THE POWER OF HYDROGEN / THE POTENTIAL OF HYDROGEN.
An ion is an atom or group of atoms that carries a positive or negative electrical charge.
pH is simply the electrochemical effect we get when negative ions (alkaline-forming) and positive ions (acid-forming) interact with each other.
pH is measured in “logarithmic units,” like the Richter scale, which measures earthquakes.
Each number represents a 10-fold change in the acidity/alkalinity of the water.
So a pH reading of 5 is ten times more acidic than a pH reading of six and 100 times more acidic than a neutral reading of 7.
Mark these words… the science of pH or the potency of health with regard to the acidity or the alkaline terrain of your body’s waters will be the new frontier in human health within the coming decade. It’s so obvious, it’s ridiculous!
Your body is made of water.
So if the ‘waters that make up your body’ are out of pH balance (as per design), then the chances are very good you will experience the collapse of your body.
Where and how it collapses is different for everyone.
Call it disease, call it aging, call it a bad knee; the waters of your body governs it all.
How pH is measured
As said above, pH measures the concentration of hydrogen ions in water.
An ion is an atom or molecule that has gained or lost electrons, and thus has a negative or positive charge.
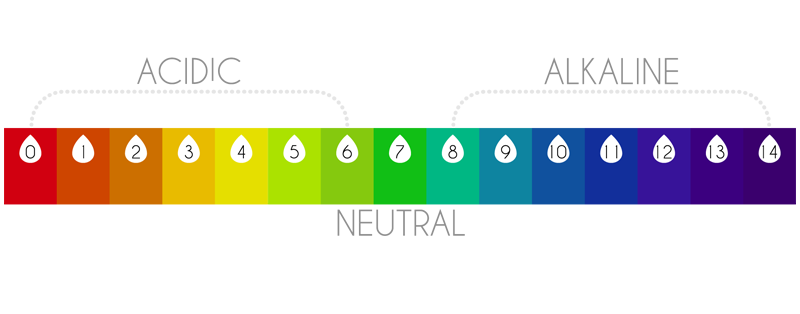
The pH scale measures the concentration of those charges, assigning them a value from 0 to 14.
Pure water at room temperature (77 degrees Fahrenheit), for example, has a pH of 7.0 and is considered neutral.
Water with a pH below 7 is defined as acidic and above 7 alkaline.
Thus, a pH measurement of 0 represents the strongest acid, and a pH value of 14 represents the strongest alkaline.
The pH scale
There is no understating the importance of the pH scale.
Created by Danish chemist S. P. L. Sorensen some 100 years ago, this simple mathematical formula measures the power of hydrogen in a solution and defines, in numeric terms, the properties of water.
Sorensens system is logarithmic.
As a result, each whole pH value below 7 is 10 times more acidic than the next higher value.
For example…
pH 4, is 10 times more acidic, than pH 5, and 100 times (10 x 10) more acidic than pH 6.
The same holds true for pH values higher than 7, each of which is 10 times more alkaline than the next lower whole value.
pH 10, is 10 times more alkaline, than pH 9, and 100 times (10 x 10) more alkaline than pH 8.
Today, in laboratories all over the world, Sorensens pH scale is used to measure the strength or weakness of acids and alkalis, the most important chemical compounds used by chemists and scientists.
An accurate pH measurement is the starting point for just about all scientific research and experimentation.
pH and you
On a practical level, pH values impact our lives in so many ways that we may not realise or think about very often.
From the food we eat to the water we drink, pH measurement is critical.
The farmers of the foods we eat rely on pH testing to maintain quality soil that will produce the most abundant and healthy crops.
pH testing is also critical in the management and safe disposal of waste water produced by industrial plants.
Without accurate pH testing, it would be impossible to mitigate the effects of pollution such as acid rain, leaving our entire ecosystem at risk.
Indeed, a diet rich in fresh fruit, vegetables, roots, nuts and legumes (that is slightly alkaline), promotes wellness and may help ward off disease.
You blood is made of water and is slightly alkaline with a normal pH level between 7.35 and 7.45.
Maintaining cellular alkalinity is the reason for understanding what you eat, drink and even think!



